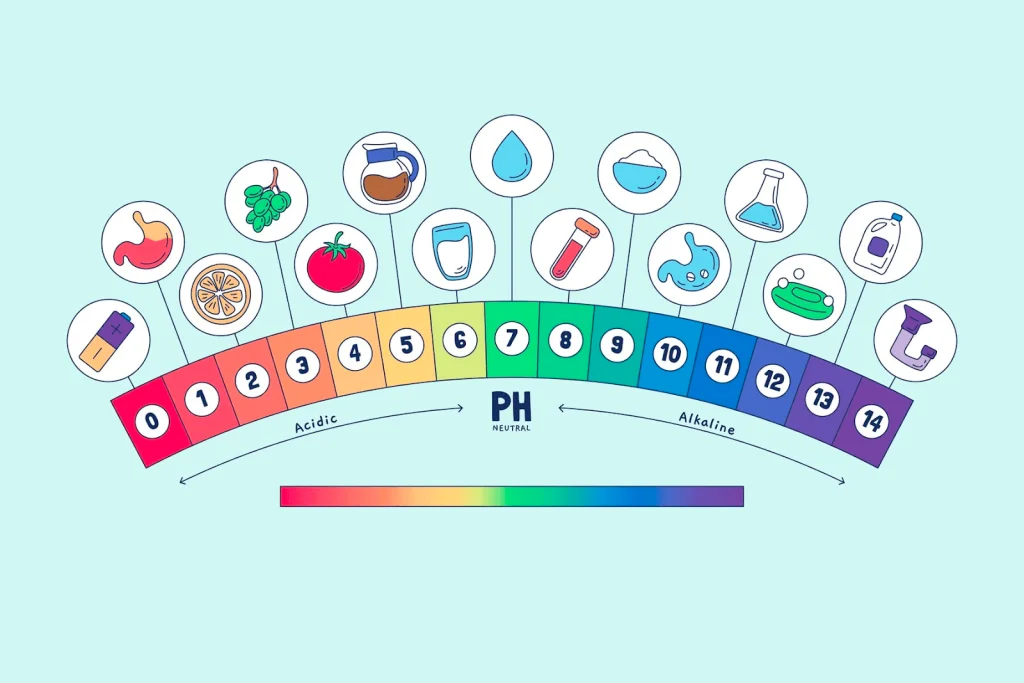
The pH level is a key indicator of water quality and safety. It measures how acidic or alkaline a solution is on a scale from 0 to 14. Distilled water is one of the purest forms of water available. With most impurities removed through the distillation process, it has many uses including scientific experiments, medical applications, and consumer products. But what is pH of distilled water? Understanding the neutral pH level of distilled water is important for those applications requiring highly pure water.
The VEVOR Water Distiller produces high-quality distilled water while efficiently removing dissolved solids. Keep reading to learn more about what is the pH level of Distilled Water and how it impacts the water’s properties for various uses. Discover why monitoring the distilled water pH range is so important.
Table of contents
Understanding pH: The Science Explained

The pH level is a measure of how acidic or alkaline a solution is. It ranges from 0 to 14, with 0 representing maximum acidity and 14 representing maximum alkalinity. A pH of 7 is considered neutral.
pH is determined by the concentration of hydrogen (H+) and hydroxide (OH-) ions in water. Acids release H+ ions which lower pH, while bases release OH- ions which raise pH.
The pH scale is logarithmic, so each incremental pH change represents a 10-fold change in acidity or alkalinity. Even small pH differences can significantly impact chemical behaviors and processes.
pH is measured using various methods. Simple pH test strips provide a rough estimate, while electronic pH meters give the most accurate readings. pH indicators change color at different pH levels, also allowing visual pH determination.
Monitoring the pH level of distilled water is critical for water quality. Extremely high or low pH levels make water unsafe for human consumption and use. For scientific and industrial applications, precise pH control is often essential.
The pH of Distilled Water: A Deep Dive

Theoretically, pure distilled water should have a perfectly neutral pH of 7.0. This is because the distillation process removes all solutes, minerals, and ions that could shift the pH up or down. Without any hydrogen (H+) or hydroxide (OH-) ions added, distilled water in its purest form neither favors acids or bases and remains neutral.
However, freshly distilled water exposed to air tends to absorb some atmospheric carbon dioxide. This dissolves to create carbonic acid, releasing hydrogen ions and lowering the pH slightly. Most distilled water right after distillation has a pH of around 5.8 due to this carbon dioxide absorption. Over time, the absorption continues until an equilibrium is reached, usually resulting in a pH between 5.7 and 7.0.
Compared to other common water types, distilled water’s typical pH range remains more neutral:
- Tap water: Ranges from approximately 6.5 to 8.5 due to minerals and added chemicals like chlorine.
- Spring water: Usually has a pH of around 7 to 8 owing to the presence of some dissolved solids picked up from rock and soil.
- Alkaline water: Often tests between 8 and 9 in pH due to added mineral ions like calcium and magnesium that increase alkalinity.
So while not completely neutral at 7.0, distilled water is closer to an ideal neutral pH than most natural and treated water sources you would encounter. This makes it desirable for applications where a neutral pH is required.
Several factors can alter or shift the pH level of distilled water away from neutral:
- Exposure to air – Absorbing carbon dioxide from the atmosphere will steadily lower pH over time after distillation. This effect is accelerated at higher temperatures.
- Storage temperature – Warmer water temperatures speed up the absorption of atmospheric carbon dioxide compared to colder storage.
- Container material interactions – Certain vessel materials like glass can leach ions into the water gradually changing its pH up or down. Stainless steel or polypropylene plastic are more inert.
- Added solutes or substances – Any additional ions, acids, bases or other solutes introduced will impact the pH accordingly.
Careful handling and storage of distilled water is required to maintain a fresh neutral pH for critical scientific and medical applications. Frequent pH testing and regulation is key to keep the pH ideal. Storage vessels should be chemically inert and kept sealed and cool.
The target pH for distilled water depends greatly on the intended usage. For general drinking purposes, a pH between 6.5 and 7.5 is recommended. However, sensitive laboratory experiments may require tighter control at 7.0 ±0.2 pH to meet methodology standards. Understanding your specific pH needs is vital when working with distilled water.
The Impact of pH on Health and Usage: Why pH Matters
The pH of water impacts many aspects of its usage for drinking, cooking, scientific experiments, medical applications, and industrial processes. Proper pH balance is crucial.
For general health, drinking water should have a pH between 6.5 and 8.5. Too low or high can harm cells and organs over time. Most bottled waters fall in a safe pH range, but testing is prudent for long-term consumption.
In cooking, water pH alters food textures and flavors. Acidic water can give foods a tangy taste, while alkaline water makes preparations more mellow. The ideal pH depends on the meal.
For science and medicine, distilled water’s neutral pH makes it invaluable. Sensitive chemical reactions rely on a controlled pH of around 7 to proceed properly. Using distilled water ensures experiments start from a neutral baseline.
In pharmaceuticals, distilled water is used to adjust pH in liquid medicines and injections. Its purity prevents contamination. A precise pH is critical for effective drug delivery in the body.
For cosmetics and skin care, mineral-free distilled water won’t irritate sensitive skin types. Its clean pH provides stability for product ingredients like preservatives and vitamins.
In industrial contexts, boilers and cooling towers depend on controlled water pH to prevent metal corrosion and scale buildup. Distilled water’s predictable pH reduces equipment damage.
So from drinking to chemistry labs, the pH level of distilled water matters. While never exactly 7.0, its expected near-neutral range allows it to uniquely maintain purity and consistency across many demanding applications. Monitoring and managing pH is essential for optimizing results.
VEVOR Water Distiller: Ensuring Optimal pH Levels
The VEVOR Water Distiller utilizes an efficient steam distillation process to produce fresh distilled water with an ideal neutral pH.
The distiller boils tap water, converting it to steam that rises and passes through a condenser coil. Pure condensed distilled water drips out, leaving behind insoluble impurities and dissolved solids.
This process removes all ions and minerals that could alter pH. The freshly distilled water comes out with a neutral pH around 5.8. Proper sealed storage keeps CO2 absorption minimal.
The VEVOR distiller has several features to optimize pH levels:
- Stainless steel construction – Inert material won’t leach ions into water.
- Automatic shut-off – Prevents overheating that could decrease pH.
- Insulated cooling system – Condenses steam faster to limit air exposure and maintain neutral pH.
Benefits of the VEVOR Water Distiller include:
- Adjustable steam rate for custom distilled water pH.
- Produces 4 liters of pure distilled water per hour.
- Carbon post-filter stage further removes impurities.
- Simple to operate with a fully automatic process.
- Safe and energy efficient.
For labs, medical facilities, and businesses that require pH-controlled distilled water, the VEVOR Water Distiller is an excellent solution.
FAQs: Addressing Common Queries About pH and Distilled Water
Q: What pH is distilled water?
A: The pH of freshly distilled water is close to neutral, around 5.8. It may range from 5.7 to 7 after absorbing some atmospheric CO2.
Q: Is distilled water acid or alkaline?
A: Distilled water is neither acidic nor alkaline. Without any ions added, it remains neutral and does not favor acids or bases.
Q: How does distilled water’s pH compare to tap water?
A: Tap water tends to be slightly acidic, with a pH between 6.5 and 8.5. Distilled water is closer to a neutral 7 pH.
Q: Why does distilled water’s pH matter?
A: Precise pH control is critical for medical, scientific, and industrial applications relying on distilled water for purity.
Q: What factors affect the pH balance of distilled water?
A: Exposure to air, storage temperature, water container material, and any added solutes can alter pH over time.
Q: How can I lower or raise the pH of my distilled water?
A: You can adjust pH using dilute acid or base solutions. But this will impact purity, so precautions are needed.
Q: What is the ideal pH range for drinking distilled water?
A: For drinking, distilled water should have a pH between 6.5 and 7.5 for health and proper hydration.
Final Words: The Role of VEVOR in Perfecting pH Balance
Understanding what is the pH of distilled water is crucial for those who rely on its purity and neutrality. While theoretically 7.0, distilled water’s true pH ranges from 5.7 to 7 after absorbing atmospheric carbon dioxide. This slightly acidic yet near-neutral pH makes distilled water ideal for scientific experiments, medical applications, and other sensitive uses where precise pH control matters.
From keeping lab tests consistent to ensuring injections are safe, monitoring and managing the pH balance of distilled water is key.
With features to optimize the steam distillation process, the VEVOR Water Distilled pH produces freshly distilled water with a neutral 5.8 pH. For laboratories, hospitals, and researchers seeking the highest quality distilled water with a reliably controlled and consistent pH, the VEVOR Distiller is an excellent choice. Achieve optimal pH levels for all your applications with VEVOR.





